The cost and throughput bottlenecks in the current monoclonal antibody platform manufacturing process are the Protein A column chromatography capture step1, which is limited in capacity and volumetric throughput and cannot be performed in a fully continuous mode. To relieve these bottlenecks, we have developed a fully continuous capture step based on precipitation paired with microfiltration2as a drop-in replacement for the Protein A-based capture step and have and demonstrated its performance with industrial monoclonal antibody feeds3. The selection of precipitation for the capture step is driven by increasing monoclonal antibody titers produced in the upstream process. As monoclonal antibody titers now routinely exceed 5 g/L, bulk separation techniques such as precipitation become more attractive relative to adsorptive separations such as bind-and-elute chromatography. Further, as the solubility of the monoclonal antibody is set by fixed concentrations of precipitants, raw material usage is essentially independent of titer.
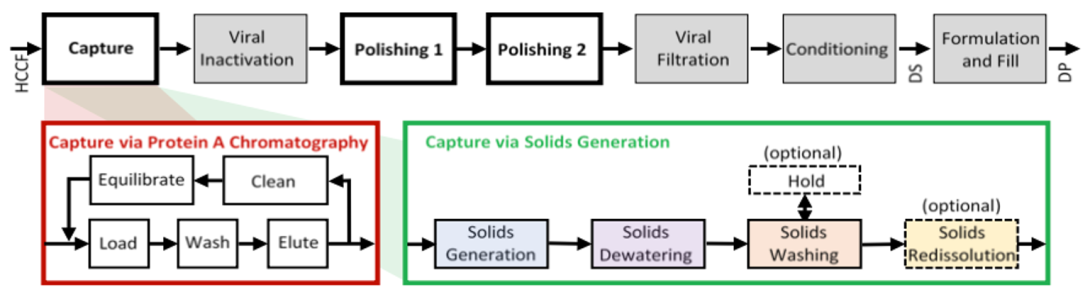
We perform monoclonal antibody precipitation with inexpensive, reversible, and non-denaturing precipitants (ZnCl2 and PEG-3350) in low-complexity continuous-flow tubular reactors with good selectivity and without capacity limitations. Continuous precipitation in a tubular reactor is the preferred format as the fluid shear history of the process stream determines the morphology of the precipitates. Tubular reactors offer well-defined fluid shear fields and narrow residence time distributions that are highly reproducible, facilitating scale-up. Precipitate generation is followed by a dewatering step to remove supernatant, one or more washing steps to remove residual supernatant and adherent impurities, and a re-dissolution step. We conduct the dewatering and washing steps in a fully continuous fashion using inexpensive static mixer/microfiltration module pairs. We can conduct the re-dissolution step at arbitrary concentrations up to the intrinsic solubility limit of the monoclonal antibody, resulting in highly concentrated, low volume process streams that can be purified in subsequent downstream processing steps with much smaller footprints.
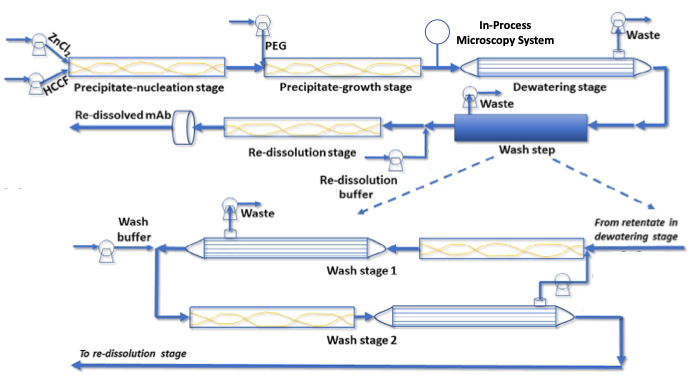
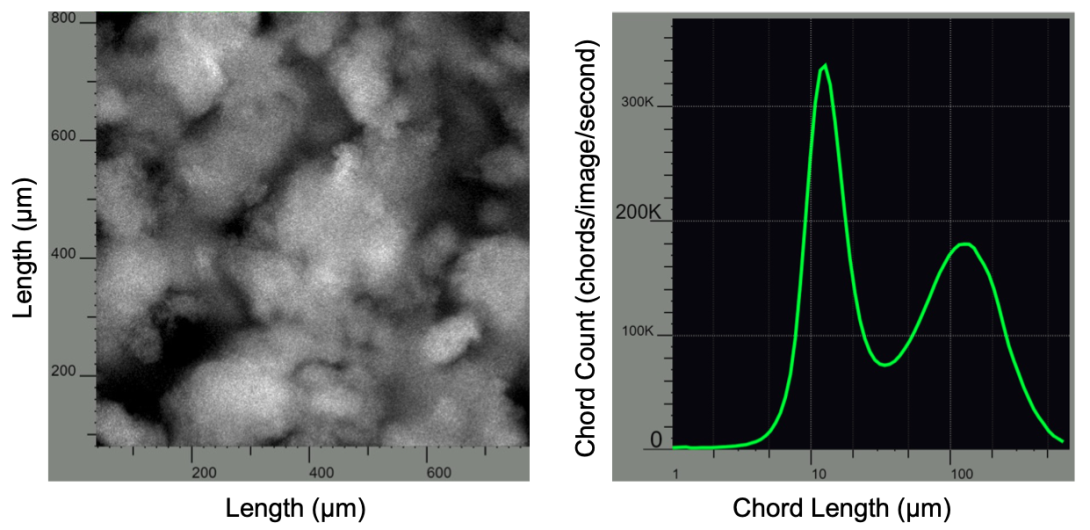
To facilitate continuous capture via precipitation for prolonged durations, we have identified precipitation operating conditions and flow formats that produce compact, high fractal dimension precipitate particles that minimize fouling of the dewatering and washing microfiltration modules. We utilize in-process microscopy to monitor precipitate particle morphology and automated image analysis to determine particle size distribution and fractal dimension. We also employ feedback control based on real-time, in-process particle morphology measurements to ensure the continuous generation of precipitate particles with properties that enable consistent, robust microfiltration performance4.
The fully continuous, eminently scalable precipitation-based capture step is a drop-in replacement for the Protein A-based capture step of the current monoclonal antibody platform manufacturing process. We have coupled the precipitation-based capture step with two orthogonal tandem flowthrough chromatography steps in a fully continuous monoclonal antibody purification process. This process is compatible with current continuous upstream processes based on staggered fed-batch or perfusion bioreactors and with continuous operation of the remaining downstream process, including viral inactivation, viral filtration, and ultrafiltration/diafiltration conditioning steps.
Selected Reference:
- Zhu, Y.; Mergy, M.; Cramer, S. M.; Przybycien, T. M. Towards Platformization of Monoclonal Antibody Capture Precipitation Solution Conditions in the PEG-Zinc Precipitant System. Separation and Purification Technology 2025, 361, 131138. https://doi.org/10.1016/j.seppur.2024.131138.
- M. Minervini, M. Mergy, Y. Zhu, M.A. Gutierrez Diaz, C. Pointer, O. Shinkazh, S. F. Oppenheim, S.M. Cramer, T.M. Przybycien, A.L. Zydney, Continuous precipitation-filtration process for initial capture of a monoclonal antibody product using a four-stage countercurrent hollow fiber membrane washing step, Biotech. Bioeng. (2023) bit.28525, https://doi.org/10.1002/bit.28525.
- Ghosh, S.; Mergy, M.; Minervini, M.; Okpanum, J.; Cramer, S. M.; Bequette, B. W.; Zydney, A. L.; Przybycien, T. M. Smart Manufacturing Implementation of a Continuous Downstream Precipitation and Filtration Process for Antibody Purification. Smart and Sustainable Manufacturing Systems 2023, 7 (1), 129–147. https://doi.org/10.1520/SSMS20230003.
- Gu, Q.; Li, Z.; Coffman, J. L.; Przybycien, T. M.; Zydney, A. L. High Throughput Solubility and Redissolution Screening for Antibody Purification via Combined PEG and Zinc Chloride Precipitation. Biotechnol Progress 2020, 36 (6). https://doi.org/10.1002/btpr.3041.