AAV Affinity Chromatography
Affinity chromatography has been a popular method of choice to capture AAVs in a manufacturing process. We employed affinity chromatography using select commercial AAV affinity columns to study the purification behavior of model AAVs with differing capsid chemistries. A column reuse affinity purification process using clarified lysate was developed to understand the impact of column reusability and process impurities on the product quality of the AAV vectors.
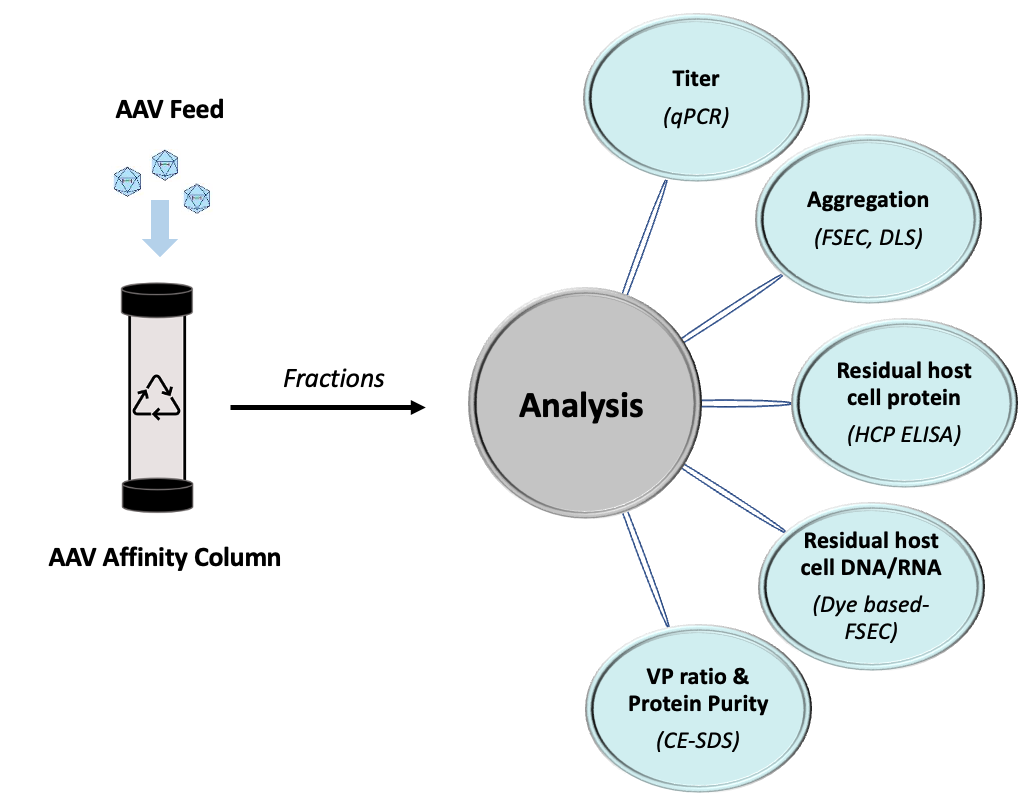
AAV Product Quality
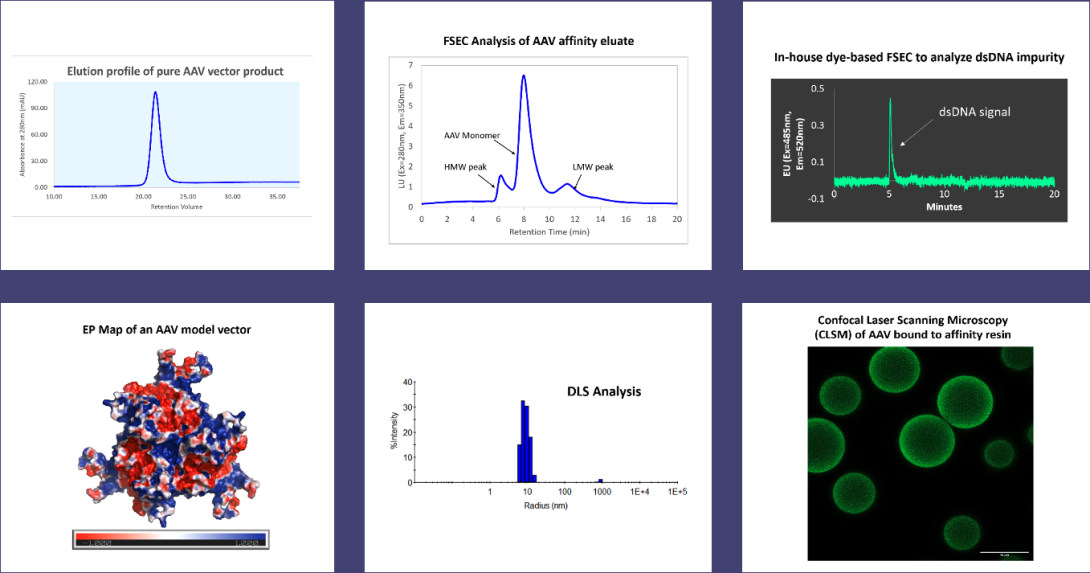
Maintaining a consistent product quality in a column reuse process is crucial. To study the effect of the affinity capture process and process impurities on our AAV vectors, a combination of analytical tools, that included analytical fluorescence-based size-exclusion chromatography (FSEC), dynamic light scattering (DLS), Dye-based SEC, HCP ELISAs, among others, were employed to characterize the quality of affinity eluate.
Column Fouling
The fouling of the AAV affinity columns and the effectiveness of the CIP protocols are being evaluated using CLSM.