Research into Lentiviral Vector (LVV) downstream bioprocessing involves investigating a set of orthogonal stationary phases to obtain high infectious recovery while maintaining colloidal stability. We are seeking to gain insight into the relationship between the process conditions used for each chromatographic separation and its impact on colloidal and particle stabilities. Colloidal stability is a critical quality attribute, as dramatic changes in LVV concentration and vector function can result from solution instabilities.
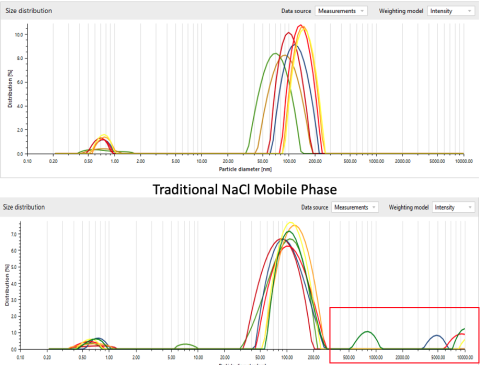
AEX LGE Fractions: Mobile Phase modified for increased stability
NaCl is conventionally used to elute LVV on AEX chromatography. Through obtaining DLS (dynamic light scattering) intensity traces of linear gradient elution (LGE) fractions, we are able to evaluate the particle size distribution of the entire spectrum of eluted particles. We’ve demonstrated that by introducing mobile phase modifiers, we can obtain enhanced colloidal stability and resolution in particle sizes.
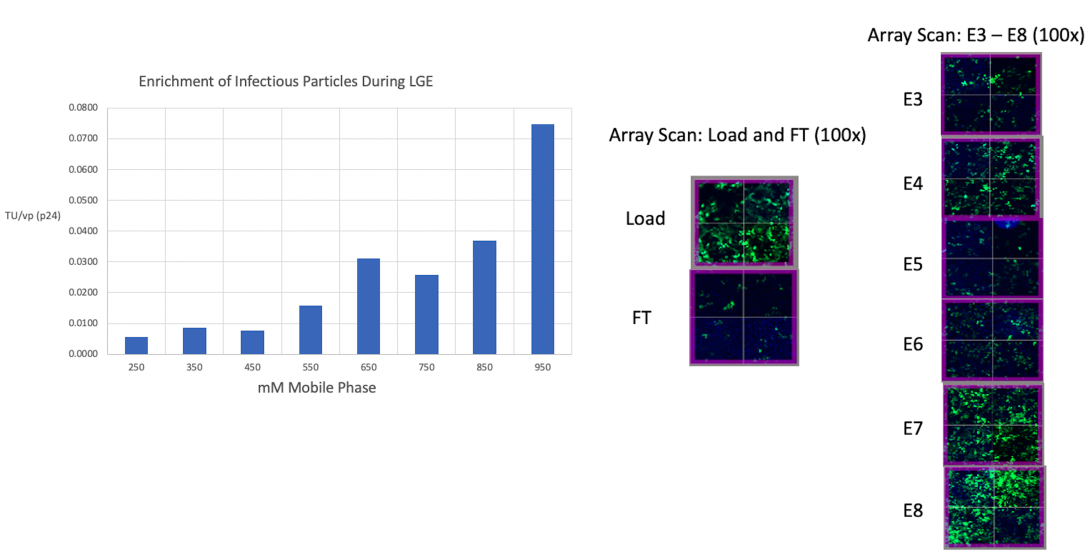
Infectivity analysis of the LGE reveals an enrichment of infectious particles which desorb at higher salt concentrations. The infectivity of individual LGE fractions is assessed by measuring the fluorescence intensity of Green Fluorescent Protein (GFP). This protein is expressed by Human Embryonic Kidney (HEK) cells once they have been transduced with purified LVV.
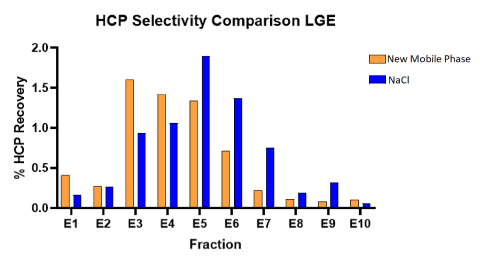
Mobile Phase Changes Impact AEX Selectivity
Investigation into AEX mobile phases allows for greater selectivity between process related impurities and infectious viral vector particles. Compared to conventionally used NaCl, new mobile phases allow for greater selectivity of host cell proteins. We are also exploring the changes in selectivity of other process related impurities such as exosomes and non-infectious viral particles.
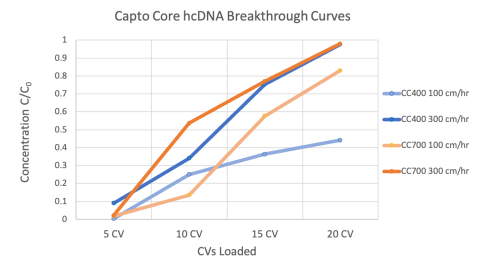
Capto Core DNA Clearance
Capto Core resins are a unique stationary phase for LVV purification, as the pore size excludes viral particles while allowing for diffusion and adsorption of host cell DNA and protein impurities. We’re assessing how varying the mobile phase, pore size distribution, and flow rate impact the recovery and purity.
Selected References:
- Ghosh, R., Koley, S., Gopal, S., Rodrigues, A. L., Dordick, J. S., & Cramer, S. M. (2022). Evaluation of lentiviral vector stability and development of ion exchange purification processes. Biotechnology Progress, 38(6), e3286. https://doi.org/10.1002/btpr.3286
- Gopal, S., Osborne, A. E., Hock, L., Zemianek, J., Fang, K., Gee, G., Ghosh, R., McNally, D., Cramer, S. M., & Dordick, J. S. (2021). Advancing a rapid, high throughput screening platform for optimization of lentivirus production. Biotechnology Journal, 16(10), 2000621. https://doi.org/10.1002/biot.202000621
- Moreira, A. S., Cavaco, D. G., Faria, T. Q., Alves, P. M., T. Carrondo, M. J., & Peixoto, C. (2021). Advances in Lentivirus Purification. Biotechnology Journal, 16(1), 2000019. https://doi.org/10.1002/biot.202000019