During the production of IgG-like bispecific antibodies numerous product related impurities are formed. These impurities have similar surface properties to the desired product. As a consequence, single mode chromatography lacks the selectivity required to achieve both high yield and high purity of the product bsAb. Mixed mode chromatography has demonstrated improved selectivity relative to single mode resins like IEX and HIC.
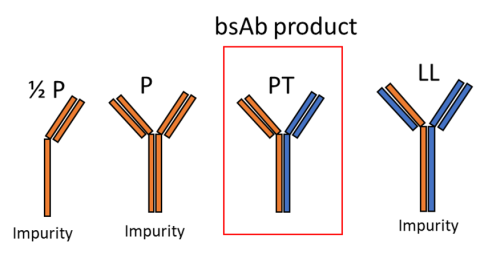
Bispecific mAb product
During the production of IgG-like bispecific antibodies numerous product related impurities are formed(1). These impurities have similar surface properties to the desired product. As a consequence, single mode chromatography lacks the selectivity required to achieve both high yield and high purity of the product bsAb. Mixed mode chromatography has demonstrated improved selectivity relative to single mode resins like IEX and HIC(2).
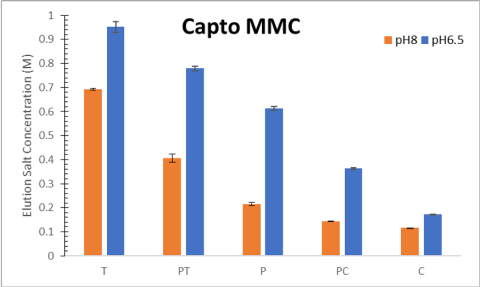
Difference in selectivity between single mode and mixed mode chromatography
These charts demonstrate the difference in selectivity between single mode and mixed mode chromatography. By comparing the first moments of the parental antibodies on these different resins, we can see a significant improvement in selectivity for the mixed mode resins(2).
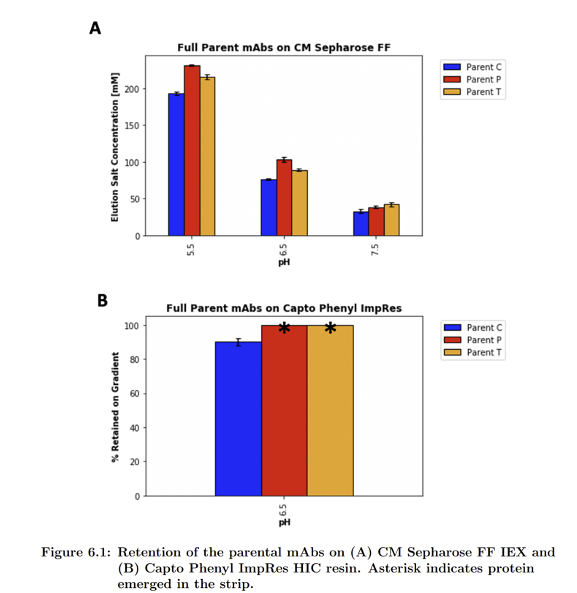
Retentions of the parental mAbs
Understanding the fundamentals of interaction between antibodies and mixed mode ligands will be useful for process development, in-silico modeling and improved ligand/antibody design. We use enzymatic digests and chromatographic screening to gauge domain contributions of the parental and bispecific antibodies on mixed mode chromatography resins. Electrostatic potential maps and surface aggregation propensity maps are used in concert with covalent labeling data to determine the binding patch on the antibody’s surface(3).
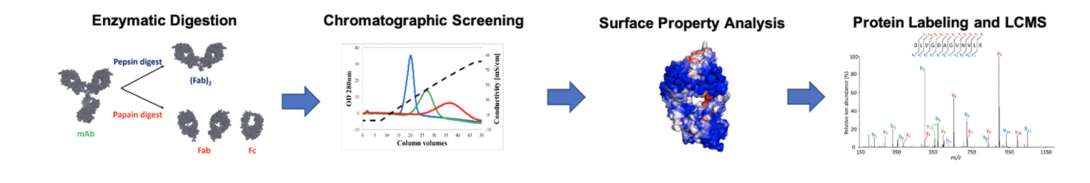
Approach Summary
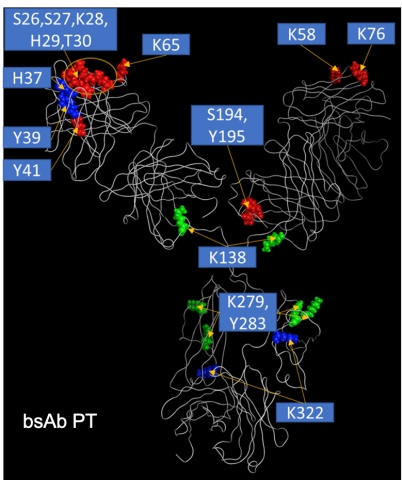
DEPC labeling
Diethyl Pyrocarbonate (DEPC) is a covalent label capable of modifying lysine, histidine, cysteine, serine, tyrosine and threonine on the antibodies surface. By comparing surface modifications of the antibody in the presence and absence of MMC resins, we can determine the binding patch on the antibodies surface. We perform the same process for the product related impurities. This allows us to compare the binding domains of different antibodies and their impurities over a range of ligands and binding conditions(2).
Selected Reference:
1.Brinkmann, U.; Kontermann, R. E. The Making of Bispecific Antibodies. mAbs2017, 9 (2), 182–212.
2.Parasnavis, S. S.; Niu, B.; Aspelund, M.; Chung, W. K.; Snyder, M.; Cramer, S. M. Systematic Workflow for Studying Domain Contributions of Bispecific Antibodies to Selectivity in Multimodal Chromatography. Biotechnol. Bioeng. 2022, 119 (1), 211–225.
3.Parasnavis, Siddharth S. Insights into the Purification of Bispecific Antibodies on Multimodal Systems: From Chromatography to Biophysics. Diss. Rensselaer Polytechnic Institute, Troy, NY, 2021.