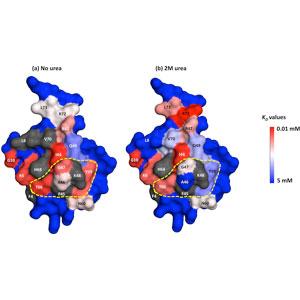
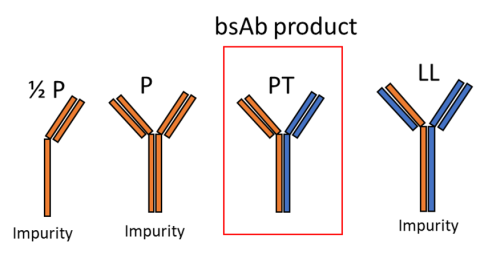
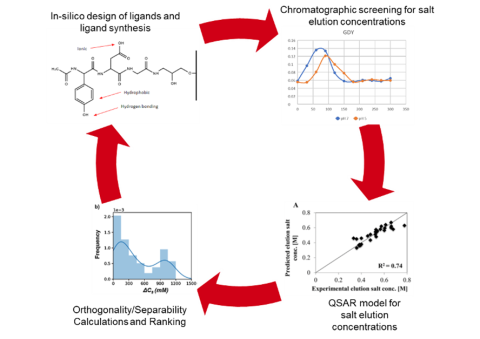
This research employs chromatographic studies with and ligand and protein libraries to provide insights into the design of novel chromatographic systems.
High throughput and bench top chromatography are employed to study the retention behavior of various separation systems and separation problems. NMR and covalent labelling/enzymatic digest/mass spectrometry are used to determine preferred binding domains of proteins interacting with various chromatographic surfaces.