Affinity precipitation is an attractive alternative to traditional affinity chromatography with limitations of high cost and complex scale-up, by exploiting a simple environmental trigger and the specific binding between affinity ligands and target biomacromolecules.
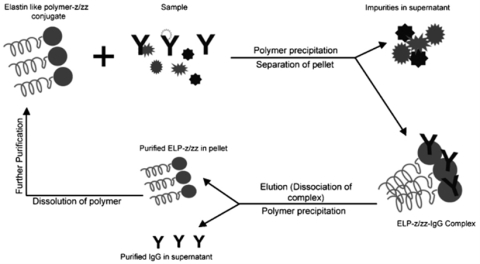
Figure 1 - Madan et al. Journal of Biotechnology 2013, 163 (1)
Chen and co-workers have fused the thermal responsive biopolymer, elastin-like-polypeptide (ELP), with a short synthetic IgG binding domain (z-domain and divalent form zz-domain) derived from the B-domain of SpA, as the capturing scaffolds for the direct purification and recovery of antibodies, Figure 11. This approach with ELP-zz has demonstrated close to 100% monoclonal antibodies recovery from hybridoma culture supernatant1.

Figure 2 - Sheth et al. Biotechnol. Bioeng. 2013, 110 (10)
We later developed a high-throughput screening method to investigate the effect of a variety of operating conditions and the performance of ELP-Z on antibody yield, purity and quality at various stages of the process and to rapidly develop a robust and high yield process, Figure 22.
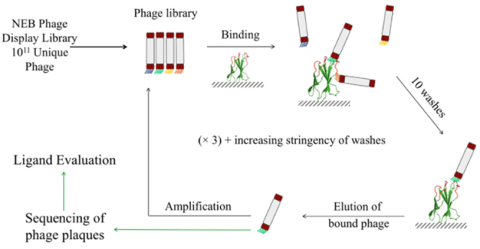
Figure 3 – Mullerpatan et al. Biotechnology and Bioengineering 2020, 117 (12)
We have then expanded this strategy to the development of an ELP-peptide fusion for the single-step affinity precipitation of the therapeutically relevant small non-mAb biologics3. Peptide ligands were first identified using phage biopanning4, as shown in Figure 33. Then lead candidate peptides were fused with ELP to evaluate their performances in affinity purification of target proteins, upon the addition of various salt solutions, as shown in Figure 45.
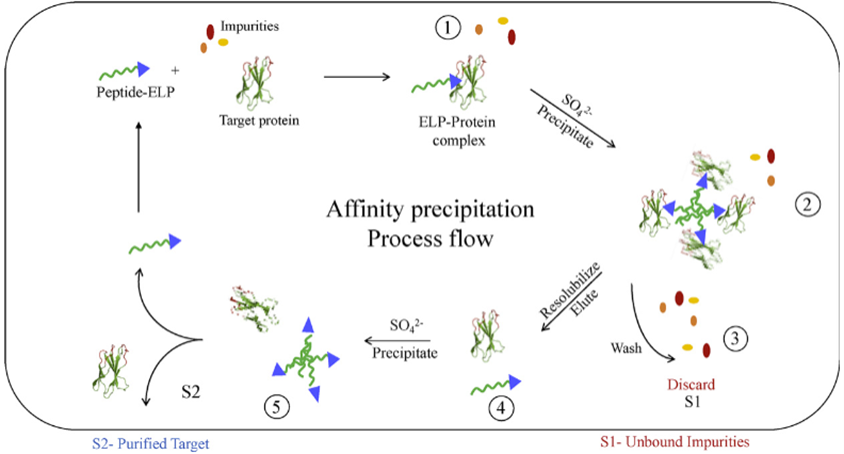
Figure 4 – Mullerpatan et al. Journal of Biotechnology2020, 309, 59–67.
Selected Reference:
(1) Madan, B.; Chaudhary, G.; Cramer, S. M.; Chen, W. ELP-z and ELP-Zz Capturing Scaffolds for the Purification of Immunoglobulins by Affinity Precipitation. Journal of Biotechnology 2013, 163 (1), 10–16. https://doi.org/10.1016/j.jbiotec.2012.10.007.
(2) Sheth, R. D.; Madan, B.; Chen, W.; Cramer, S. M. High-Throughput Screening for the Development of a Monoclonal Antibody Affinity Precipitation Step Using ELP-z Stimuli Responsive Biopolymers: HTS for Monoclonal Antibody Affinity Precipitation. Biotechnol. Bioeng. 2013, 110 (10), 2664–2676. https://doi.org/10.1002/bit.24945.
(3) Mullerpatan, A.; Kane, E.; Ghosh, R.; Nascimento, A.; Andersen, H.; Cramer, S.; Karande, P. Single‐step Purification of a Small Non‐mAb Biologic by Peptide‐ELP‐based Affinity Precipitation. Biotechnology and Bioengineering 2020, 117 (12), 3775–3784. https://doi.org/10.1002/bit.27539.
(4) Nascimento, A.; Mullerpatan, A.; Azevedo, A. M.; Karande, P.; Cramer, S. Development of Phage Biopanning Strategies to Identify Affinity Peptide Ligands for Kappa Light Chain Fab Fragments. Biotechnol. Prog. 2019, 35 (6). https://doi.org/10.1002/btpr.2884.
(5) Mullerpatan, A.; Chandra, D.; Kane, E.; Karande, P.; Cramer, S. Purification of Proteins Using Peptide-ELP Based Affinity Precipitation. Journal of Biotechnology 2020, 309, 59–67. https://doi.org/10.1016/j.jbiotec.2019.12.012.